When Risk Becomes Reality
In specialty pharma, every degree matters—and every delay has a cost. As products become more targeted and high-value—such as cell therapies, controlled medications, and rare biologics—the demand for secure, real-time-monitored cold chain storage and logistics has never been greater.
At the same time, the shift of biologics off-patent and the near-shoring of API sourcing and manufacturing are driving the need for 3PLs that can safeguard raw material integrity from import through final formulation.
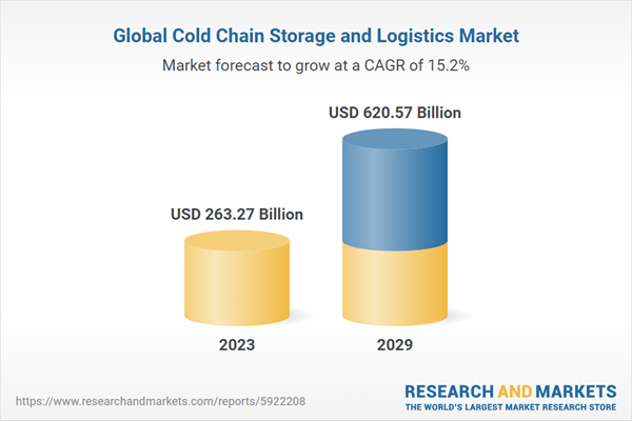
Global Cold Chain Storage and Logistics Market – Source: Research and Markets
More than 85% of biologics require cold storage (SCMR), making the stakes for precision and reliability extremely high. Meeting this demand calls for third-party logistics partners with a powerful combination of capabilities: advanced security and compliance programs, broad temperature range support, and technology-enabled supply chain visibility.
In this article, we’ll examine each of these capabilities and explain why they are critical to building a resilient, compliant, and cost-effective pharma distribution strategy.
Cold Chain Security in an Era of High-Stakes Therapies
Specialty pharma organizations primarily handle biologics, controlled substances, and cell & gene therapies (CGTs). These assets are not only temperature-sensitive—they’re often theft-prone, mission-critical, and highly regulated.
Since most 3PLs serve a wide variety of industries, the security structures they have available are not sufficient to protect the extra vulnerable and critical materials that the healthcare industry relies on. In contrast, 3PLs that exclusively serve healthcare organizations are hyper-attentive to product security and integrity every step of the way.
Looking for a shipping and storage partner with TAPA-A certified facilities is a good starting point when looking for a cold chain storage partner. Another important capability to look for is cold dock-to-cooler workflows and automated conveyance that reduce handling time and risk.
What is TAPA-A certification?
The Transported Asset Protection Association (TAPA) brings together manufacturers, logistic service providers, law enforcement agencies, insurers, and security service providers to set standards for facility and trucking security.
An approved entity must thoroughly audit 3PLs that seek TAPA certification for their facilities or transport vehicles. An organization cannot claim to be “TAPA certified” unless all its facilities and transportation assets have successfully achieved active certification.
To further differentiate between levels of protection, TAPA offers three tiers of security achievement:
- Level C = Standard Security Protection
- Level B = Moderate Security Protection
- Level A = Elevated Security Protection
Vendors who have achieved a TAPA-A mark have passed an external audit process certifying that a facility (or multiple) has the highest level of protection. When affiliated with TAPA, organizations also must prove that they conduct robust internal compliance monitoring in years between formal audits.
LSL is proud to hold a TAPA-A certification for all our warehouses. Our specialty pharma partners know our facilities are purpose built to meet the supply chain needs of healthcare products— equipped with security, surveillance, and strict access control.
Real-Time Visibility Prevents Excursions, Ensures Quality
In industries with less sensitive products, small deviations in route or temperature during transport and storage have little impact on quality or effectiveness. For pharmaceutical companies, however, those same minor changes can result in costly waste—or, worse, a compromised product that can’t be used.
Materials that require cold chain storage typically have very narrow temperature ranges, most falling into one of the following categories:
- Controlled Room Temperature (20°C to 25°C)
- Refrigerated (2°C to 8°C)
- Frozen (-20°C)
- Ultra Low Frozen (-60-80°C)
- Liquid Nitrogen (LN2) (-196°C)
Temperature excursions (variations outside of the required range) don’t just threaten product quality—they can derail an entire trial or product launch. As a manufacturer, the condition and quality of your product are extremely valuable.
In healthcare logistics, it’s crucial to monitor humidity, shock, and vibration in addition to temperature and location. These factors provide a complete picture of shipment integrity and help ensure compliance with stringent regulations like the Drug Supply Chain Security Act (DSCSA), which requires traceability of products down to the individual package or vial.
That’s why the premiere cold chain 3PLs have implemented IoT (Internet of Things)-enabled tracking. This advanced visibility allows specialty pharma companies to monitor their products across every supply chain touchpoint—from storage conditions to time spent at customs—while meeting DSCSA documentation and traceability requirements. IoT-integrated cold chain infrastructure provides continuous, real-time data on both location and environmental conditions, enabling rapid intervention before a temperature spike occurs, supporting full audit readiness, and building QA team confidence.
LSL leads the healthcare industry in IOT-enabled visibility and DSCSA compliance support. We don’t just ensure your product is in the right place, at the right time, in the right temperature range—we also provide the real-time data and traceability needed to maintain compliance from early development through final delivery.
Integrated Into Business Continuity Plans
Disruptions—whether from supply chain chaos, natural disasters, or geopolitical instability—can destroy millions in product value overnight. LSL’s cold chain is built for resilience, with redundant ultracold storage, cross-site inventory management, and visibility tools that allow for fast rerouting. Clients integrate LSL’s capabilities directly into their disaster recovery and contingency plans.
Another key way to support business continuity is to be prepared for shifts in market demand. For example, GLP-1 manufacturers often face supply shortages that demand faster manufacturing cycles and just-in-time cold chain delivery to meet on-time quality standards. Resilient supply chain planning is crucial to support GLP-1 ramp-ups, where delivery timelines are critical to addressing shortages.
Recap: Differentiators That Matter
When looking for a cold chain partner to transport, secure, store, and deliver critical medical treatments, make sure the 3PL you choose has:
- TAPA-A Certified Facilities: Controlled substance handling and global distribution security.
- Digital System Integration and IoT-Based Monitoring/DSCSA Compliance: Integrated systems and actionable, real-time temperature and location tracking to prevent loss and meet compliance demands.
- Carrier-Agnostic, Risk-Aware Logistics: Flexible courier options, allowing dynamic route and carrier decisions based on risk and speed.
- Proven BCP Integration: Seamless cold chain continuity plans, even during crises.
The Impact in Practice
LSL is proud to check every box listed above—and more. Explore a few examples of how we secure and preserve pharma products throughout the supply chain:Controlled Substances
A global CMO relied on LSL’s TAPA-A certified, DEA-compliant network to distribute pain management therapies across the U.S. Leveraging cold dock-to-cooler automation, reusable small-parcel shippers, and IoT-enabled tracking, LSL reduced theft risk by 90% while maintaining full visibility and compliance from pick-up to delivery.
CGT Commercialization
A gene therapy firm with -70°C autologous therapy requirements partnered with LSL for coast-to-coast deployment. Using chain-of-identity protocols, DSCSA-ready traceability, and IoT monitoring (temperature, location, humidity, and vibration), the firm achieved <1% temperature deviation across 600+ shipments while meeting stringent FDA timelines.
API Import Integrity
A global generics manufacturer safeguards its supply chain by using LSL’s cold chain–enabled import process to protect the integrity of APIs sourced from overseas suppliers. Each shipment is equipped with data loggers that track temperature conditions across the entire journey, ensuring materials remain within strict stability ranges. This cold chain assurance mitigates quality risks, reduces potential loss, and supports regulatory compliance while also minimizing tariff exposure by preventing rejected or compromised product.
For specialty pharma, choosing a logistics partner isn’t just about moving product—it’s about protecting it. With advanced visibility tools and unmatched security, LifeScience Logistics is redefining what it means to deliver with confidence.

