The Case for Proximity in Healthcare Manufacturing & Distribution
Modern supply chains demand more than just movement, they require velocity, value, and resilience. Co-locating warehouse and logistics operations near production sites, distribution nodes, or key markets offers strategic advantages that ripple across the entire operation.
The core benefits of localization fall in three key domains: acceleration, cost containment, and risk mitigation.
1. Supply Chain Acceleration
Positioning warehousing operations close to manufacturing or distribution hubs can drastically improve the velocity of your supply chain. In high-stakes environments, especially those involving pharmaceuticals, biologics, or other time-sensitive goods, every minute counts.
Proximity ensures goods flow rapidly between suppliers, warehouses, and production lines, eliminating bottlenecks and reducing idle time.
- Minutes (not days) to nearby production lines
- Reduces inbound and outbound lag time
- Faster deployment of finished goods and raw materials
2. Cost Containment
By optimizing for proximity, companies can significantly reduce transportation expenses and avoid costly waste associated with delays and spoilage.
Especially in sectors that require temperature control or deal with sensitive materials, proximity supports more economical and reliable ground shipping while enabling leaner, smarter inventory strategies.
- Cuts transport spending through ground optimization
- Lowers risk of spoilage with reduces transit time for APIs and biologics
- Supports Just-In-Time/Just-In-Case (JIT/JIC) hybrid models that right-size inventory levels
Did You Know?
There are 17 major national and international biopharma industry hubs that empower a localized logistics strategy. Of these 17, North Carolina’s BioPharma Crescent ranked first in the U.S. as the most cost-effective region to manufacture and fourth worldwide, along with Dublin, Tel Aviv, and Bangalore.
3. Risk Mitigation
A well-chosen inland facility can shield supply chains from many external threats, from coastal weather events to energy disruptions.
Proximity doesn’t just mean being “close,” it means being strategically located to support resilience and recovery. Choosing a site with built-in redundancies and a strong operational track record adds another layer of assurance.
- Reduces coastal storm exposure
- Ensures business continuity with 100% redundant power
- Supports safety and maintains quality of high-value inventory
Maximize Geographical Benefits with Industry-Specific Knowledge
Localization can prevent many delays, but it can’t solve for a logistics partner that doesn’t know healthcare. In this industry more than most, specialized support matters.
The demands of regulated industries like healthcare require specific infrastructure, environmental controls, and operational discipline that go far beyond traditional warehousing. A true logistics partner is not just a local storage facility; it is a deep well of healthcare knowledge and strategic guidance.
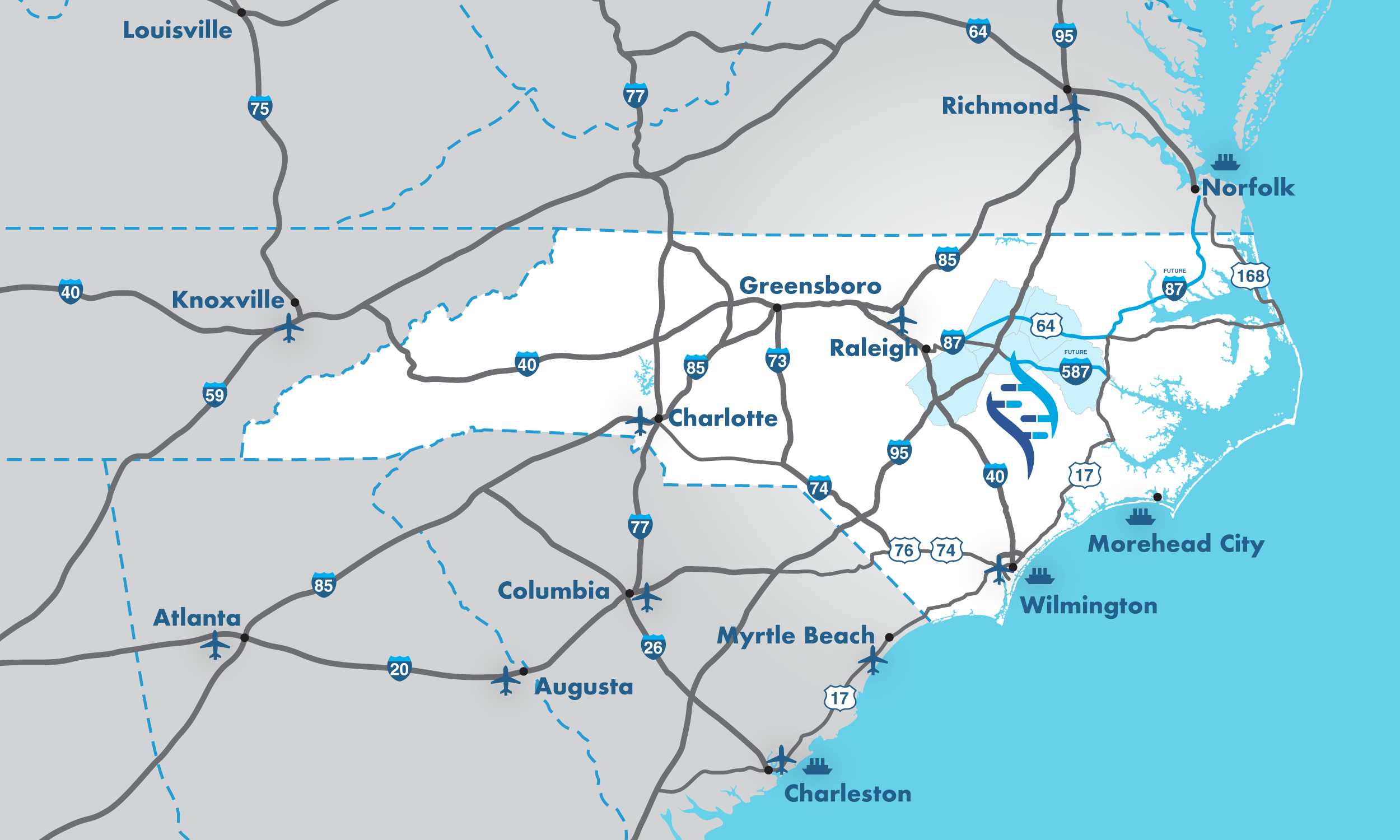
To illustrate, let’s go back to North Carolina’s BioPharma Crescent:
As the most cost-effective manufacturing region in the U.S., the BioCrescent / BioPharma Crescent corridor has rapidly become a national hub for biopharma supply chain logistics. Its central East Coast location, halfway between New York and Florida, puts 200 million consumers within a two-day drive, while deep-water ports and international airports connect RTP manufacturers to global markets.
The unique combination of industry, infrastructure, and innovation found in this region, as well as its immediate proximity to commercial-scale production facilities, contract manufacturers, and clinical trial operations, makes it an ideal location for just-in-time material staging, cold chain logistics, and integrated manufacturing support.
Research Triangle Park (RTP), the western anchor of North Carolina’s BioPharma Crescent, is one of the largest and most dynamic research and manufacturing hubs in the United States.
Spanning over 7,000 acres and home to hundreds of life science and healthcare technology companies, RTP is a magnet for healthcare innovation, scale-up manufacturing, and supply chain orchestration, bringing together world-class academic institutions, leading pharmaceutical firms, and next-generation biotech startups into a single, highly integrated ecosystem.

Nestled within this ecosystem, you’ll find LifeScience Logistics.
We built a state-of-the-art facility in the heart of the BioCrescent to be a local solution for specialized pharmaceutical and medical device needs:
- Size: 326,290 SF of GMP-compliant logistics space
- Temperature-Controlled Storage: CRT and 2–8°C cold chain support
- Humidity Control: Ideal for APIs, biologics, and sensitive materials
- Redundant Power: Full backup for unbroken cold chain protection
- Security: TAPA-certified standards for high-value inventory
- Purpose-Built: Designed for pharma, not retrofitted
Localization Meets Specialization: Use Cases In Action
Just-in-Time (JIT) and Just-in-Case (JIC) Models
Store materials near the line to reduce delays and retain flexibility.
Example: A biotech firm manufacturing cell therapies uses a hybrid JIT/JIC model. Daily delivery of buffer solutions to the facility floor ensures lean production, while an excess inventory of high-risk components is held nearby for contingency, protecting output during supplier backlogs without overburdening on-site storage.
Raw Material Staging
Pre-position APIs and packaging components with climate control to support production uptime.
Example: A specialty drug manufacturer stores its temperature-sensitive active ingredients (APIs) in a nearby GMP warehouse with 2–8°C cold chain capabilities. When a production spike occurs, the facility dispatches components within hours, avoiding costly downtime and ensuring uninterrupted batch release
Finished Goods Pull-Back
Gain storage overflow capacity without ceding control—and pull products back as needed for commercial demand.
Example: A pharmaceutical brand with limited on-site space finishes a production run of seasonal allergy medication and stages the product in a regional warehouse. As retail demand increases in specific markets, the company pulls back inventory on a controlled, as-needed basis—eliminating stockouts while maintaining centralized oversight.
Business Continuity
100% redundant power and local placement protect manufacturers from shutdowns during regional disruption.
Example: When a severe storm causes widespread power outages across the eastern seaboard, a medical device company continues shipping uninterrupted from its inland North Carolina facility. Redundant power and secure storage preserve sensitive diagnostics and uphold SLAs with hospital systems.
Clinical-to-Commercial Scale Transitions
Facility flexibility supports scale-up logistics without missing a beat.
Example: A gene therapy startup receives FDA approval and shifts from clinical batch production to commercial scale. Instead of overhauling infrastructure, they scale up logistics at their existing storage partner’s facility—moving seamlessly from small-run kitting to national distribution, all within the same validated environment.
Ready to Strengthen Your Logistics Network?
Whether you’re scaling from clinical to commercial, staging high-value raw materials, or safeguarding your cold chain during peak demand, LifeScience Logistics’ newest North Carolina facility offers the infrastructure and proximity to help you execute with speed, precision, and confidence.
Let’s discuss how LSL’s regional presence can support your supply chain goals.
We chose LSL because they had local representation and a competitive bid. We have stayed with LSL as our 3PL because they are great business partners behind the scenes and deliver great service to downstream customers with on-time delivery.
